Why Does Ocean Water Evaporate Even Below Boiling Point?
Ever wondered how water from oceans turns into rain? 🌧️
Although it does not attain the boiling temperature, water of the oceans also evaporates. A query arises about this, and it’s how and why it happens in the natural process of evaporation at temperatures far much lower than 100°C. Let’s get to know.
Water Evaporating Riddle at Lower Temps
We usually think of water vaporization as the need to boil so it can happen at 100°C (212°F). But we realize this is actually a process that can happen at much lower temperatures because of evaporation. That’s why puddles eventually dries-up after a warm-storm’s heavy rain, or why ocean water evaporates under the sun way before it reaches its boiling point. For a closer look at the behavior of water molecules and the role of energy in the process of evaporation, let’s pursue an understanding of this process.

The Science Behind Evaporation
Molecular Motion: Water is made up of billions and billions and billions of molecules, et cetera; those molecules are in motion. Even at room temperature, a few of the molecules have enough energy to escape from the liquid’s surface and become vapor in the air.
Energy and Heat: For an evaporation process to take place, a water molecule has to attain an amount of energy that allows it to overcome the attractive forces between it and neighboring water molecules, holding it in the liquid phase. This energy most of the time is attributed to heat. Literally, to this point, the rays of the sun at lower temperatures can cause an increase in the rate of evaporation of water.
Surface area matters. The greater the surface area of the water, the more molecules are exposed to the air, the less difficult it is for molecules to acquire enough energy to make it into the gas phase. This explains why shallow puddles would always dry sooner compared with deep ones.
Humidity and Air Flow: In dry and moving air, water vapor will be swept off rather quickly because it will speed up the rate of evaporation as it can accommodate more and more vapor molecules in the air. In humid condition, the evaporation is slow because air will have already been saturated with moisture.
Why Evaporation Differs from Boiling
Boiling vs Evaporation: Boiling is a rapid process of vapor formation as soon as an entire liquid reaches a certain temperature level at which it is able to transfer into gaseous form. Contrary to this, evaporation is the slower process of vapor formation, only happening at the surface of liquid, and there is no need for the whole of the liquid to reach up to its temperature of boiling.
Temperature Variation: Boiling demands that the liquid become of a specific temperature altogether (in the case of water, 100°C). Contrarily, evaporation constitutes the phenomenon where the molecules on the surface gain energy from the surroundings and evaporate at any temperature.
Energy Source: In order for evaporation to take place, it needs energy what the energy is from is immaterial (sunlight, temperature of air, and wind are a few examples). This is what explains why water can evaporate on a chilly day or in the shade.
Some Real-World Examples of Evaporation
When one hangs wet clothes out to dry, the temperature is even far below the boiling point; the clothes continue giving off water vapour. The energy is applied to the process whereby the drying occurs when energy is sufficient for the water molecules to escape to the atmosphere.
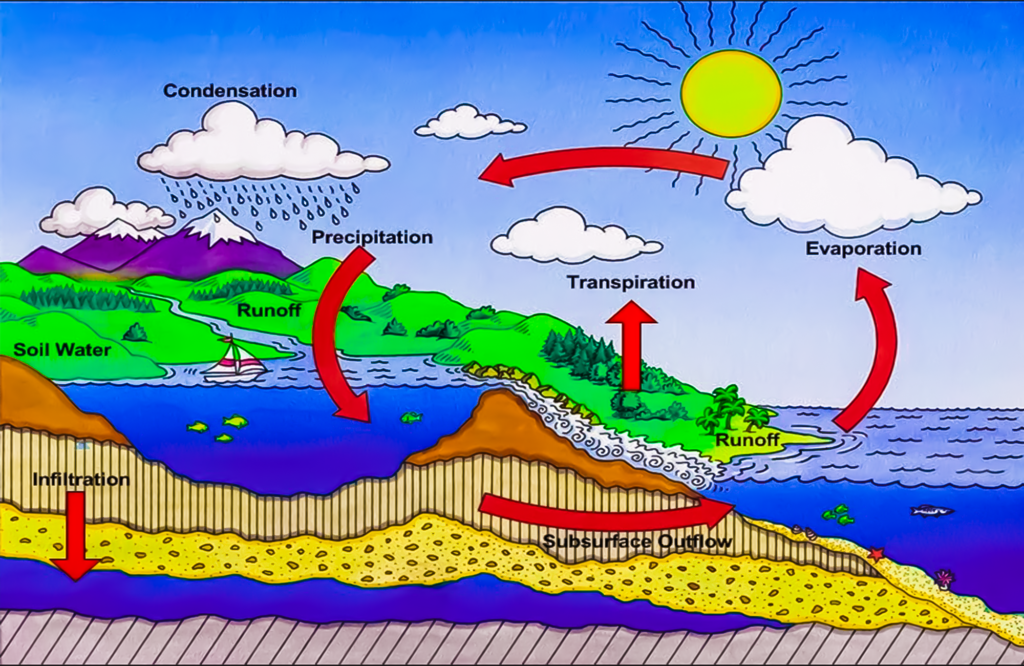
Ocean Evaporation: A large part of the earth’s surface is occupied by water bodies like oceans. The enormous expanse, seen in conjunction with solar heating, occasions a gigantic amount of coupled with solar heating. This goes into the water cycle by creating clouds, which, in turn, fall as rain.
Drying Following a Rain: The sun very effectively causes surface water to evaporate following a rain. The pool shrinks because the energy from the sun supplies its molecules with energy sufficient to escape even with a quite low final temperature.
Conclusion
It is quite an interesting process where water can transform to vapor even when the temperature is nowhere near its boiling point. This natural process is very critical in the water cycle, controls the climate, and in activities we undergo daily like drying our clothes or cloud formation. Understanding the molecular movement and energy taking place makes one understand why the water in the ocean and other water bodies evaporate even at temperatures below 100° C.
More insights into the wonders of nature!